Abstract
Dyskeratosis congenita (DC) is a telomere biology disorder caused by pathogenic germline variants in genes essential in telomere biology resulting in very short telomeres for one's age. Telomere length (TL) <1st percentile for age as measured by flow FISH is diagnostic of DC.
The novel Telomere Shortest Length Assay (TeSLA) provides information on the full distribution of telomere length (TL), including short and ultrashort telomeres, which are believed to define the biological signal of telomere-mediated replicative potential. We compared TeSLA TL parameters between patients with DC and healthy controls and assessed its relationship(s) with DC phenotypes.
The study included 19 patients with DC with a proven pathogenic germline variant who enrolled in an IRB-approved longitudinal cohort study of inherited bone marrow failure syndromes (NCT00027274); detailed questionnaires were completed and medical records were reviewed with a subset of patients being evaluated at the NIH Clinical Center. Controls (N=22) were healthy hematopoietic cell transplantation donors from the Center for International Blood and Marrow Transplant Research biorepository. DNA was isolated from blood by standard methods.
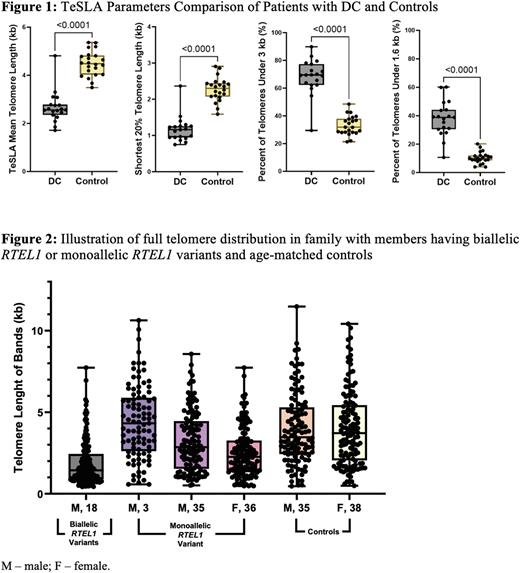
Flow FISH mean TL in lymphocytes and TeSLA mean TL were highly correlated in DC patients (R2=0.66, p<0.0001). Significant differences were noted between DC patients and healthy controls in all TeSLA parameters (Figure 1). Statistically significant associations between TeSLA ultrashort TL and DC phenotypes were noted. Specifically, a higher accumulation of ultrashort TL was observed in DC patients with severe compared with mild or moderate bone marrow failure (% TL <1.6kb=42.68% vs 26.76%, p=0.008) and in patients with multi-system compared with single-system phenotypes (43.16% vs 30.41%, p=0.02).
In a subset of families with RTEL1 variants, the accumulation of ultrashort TL was higher in patients with biallelic variants than heterozygotes or controls (% TL <1.6kb=44.99%, 23.70%, and 10.40%, respectively). Figure 2 shows TeSLA TL distribution differences in one family with members having biallelic or monoallelic RTEL1 variants and two age-matched controls.
Our findings underscore the valuable information that TeSLA may provide in the understanding of the contribution of short and ultrashort telomeres to the pathogenesis of telomere biology disorders.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal